Fibromyalgia Information & Local Support
Honours for this website
Fibromyalgia Information & Local SupportHonours for this website |
 |
Written by: David A. Nye MD
November 4, 1998
Fibromyalgia syndrome (FMS) is an under diagnosed disorder of unknown etiology affecting over 5% of the patients in a general medical practice (Campbell 1983) and an estimated 2-4% of the general population (Wolfe 1993), women more often than men. Patients complain that they ache all over. A large number of other symptoms are often present, particularly fatigue, morning stiffness, sleep disturbance, paresthesias, and headaches (see table 2). On examination, areas of focal tenderness called tender points can be demonstrated in characteristic locations (table 3). Most patients can be helped substantially with treatment.
A comprehensive review of the many theories of the etiology of FMS is beyond the scope of this paper. While there is still not a majority of FMS researchers who support any one theory, significant progress is being made in identifying an etiology, and much useful evidence has been collected.
FMS was first described as an inflammatory condition (Gowers 1904). When no evidence of inflammation could be found and an association was noted with depression and stress, the concept of "psychogenic rheumatism" was advanced (Boland 1947), but a number of studies have established that FMS is neither a psychosomatic nor somatiform disorder and that when present, anxiety and depression are more likely to be the result than the cause of FMS (Goldenberg 1989, Yunus 1991, Dunne 1995).
It has been suggested that the pain of FMS is related to microtrauma in deconditioned muscles and that exercise works by conditioning these muscles (Bennett 1989). However, reports of muscle biopsy abnormalities other than disuse atrophy have been difficult to replicate (Schroder 1993), and some tender points are not over muscles or tendons, such as the one over the medial fat pad of the knee (Smythe 1989). Muscle energy metabolism is normal in FMS (Simms 1994, Vestergaard-Poulsen 1995).
FMS may be due to non-restorative deep sleep (Moldofsky 1975, 1993). Patients with FMS often report insomnia or light sleep as well as an increase in FMS symptoms after disturbed sleep (Campbell 1983). Abnormal amounts of alpha activity on the electroencephalogram of FMS patients during deep sleep have been reported (Hauri 1973, Moldofsky 1975). FMS-like symptoms can be induced in normal volunteers by depriving them of deep sleep, except in subjects who exercise regularly (Moldofsky 1975). Controlled trials have confirmed the value of aerobic exercise in the treatment of FMS (McCain 1988). Exercise increases time spent in deep sleep (Hobson 1968), perhaps a mechanism for its therapeutic effect.
A number of changes in immune system function have been found in FMS, generally in the direction of increased activity, many of which can also be induced in normal volunteers through sleep deprivation (Moldofsky 1993). Many of the symptoms of FMS may be caused by elevations, induced by abnormal sleep, in certain cytokines such as interleukin-2, which has been found to be elevated in FMS patients, and which causes FMS-like symptoms when given intravenously (Wallace 1990, Moldofski 1995) .
Serum levels of serotonin and its dietary precursor tryptophan are low in FMS (Russell 1996). Amitriptyline, one of the medications often used to treat FMS (see below), blocks serotonin reuptake and increases deep sleep (Baldessarini 1985). Serotonin is important in deep sleep and in central and peripheral pain mechanisms (Chase 1973). Whether serotonin abnormalities are etiologically important in FMS or secondary is yet unknown.
The concentration of substance P, a peripheral pain neuro- transmitter, is several times higher in the cerebrospinal fluid of FMS patients than in pain-free controls, implying a peripheral origin for FMS pain (Russell 1994). A number of other neuroendocrine abnormalities have been identified in FMS patients (Crofford 1994, Moldofsky 1995, Russell 1996) which form the basis for other theories of the etiology of FMS.
Although no specific inheritance pattern has been identified, an increased incidence in relatives of affected patients has been noted (Pellegrino 1989). Development of the syndrome may require a predisposing factor, possibly inherited, as well as a precipitating factor such as trauma, infection, stress, or sleep disruption. The immunologic abnormalities have suggested to some an infectious etiology, but if FMS were infectious we would expect to see an increased incidence in spouses of an affected patient and this is not the case.
Since FMS is a syndromic diagnosis, any patient who fits the diagnostic criteria of chronic, diffuse aching with tenderness in at least 11 of 18 characteristic locations (Table 3) has iccurately diagnosed by exclusion. One would expect medical students to have been taught in physical diagnosis how to examine for a disorder that accounts for more than 5% of a primary care practice but lamentably this is not yet the case in most medical schools. If a patient has typical symptoms of FMS (Table 2) but does not meet the tender point criterion, a diagnosis of "possible FMS" may be assigned and a therapeutic trial of standard treatment offered. Tender points should be looked for again on a return visit as they may be more evident on some days than others.
Although there have been many abnormalities of laboratory and other tests reported in FMS, none is sufficiently sensitive nor specific to be useful diagnostically, so routine studies are not indicated. Patients who haven't recently had a general medical evaluation should as part of the workup, and other tests should be ordered when the history or exam raises a question of something other than FMS. In older patients a sedimentation rate may be useful to exclude polymyalgia rheumatica. In patients with other symptoms of hypothyroidism, thyroid studies should be done.
The current syndrome definition may not be the best one possible (Wolfe 1993). It has been argued that tender points have been over-emphasized, probably because historically rheumatologists have been more involved in the diagnosis and treatment of FMS than other specialists. In many patients who meet the criteria for diagnosis for chronic fatigue syndrome, the only difference between them and a typical FMS patient is the degree of pain. Some of these patients followed over time will subsequently develop tender points and then fit the criteria for diagnosis of FMS. 70% of patients with FMS meet the CDC criteria for CFS (Buchwald 1987) and two thirds of patients with CFS meet the ACR criteria for FMS (Goldenberg 1990b). It seems unlikely that these patients have two separate disease processes. Perhaps dividing these two groups of patients on the basis of whether or not they have prominent pain is as artificial as division on the basis of prominence of any of the other twenty or so associated symptoms.
On the other hand, we are to some extent stuck with the current syndrome definition because it is these patients on whom all the important studies have been performed. If the syndrome definition is altered, we can't be certain that all of these restill apply to the new syndrome. This problem will disappear once we know the true etiology and can make an etiologic rather than syndromic diagnosis.
Controlled studies have shown that amitriptyline (l 1991), aerobic exercise (McCain 1988), and other interventions to be discussed later are of benefit in treating FMS, but the percentage of patients responding to each alone is small. When gentle daily aerobic exercise, a consistent bed time wibeen studied rigorously, but in an unpublished retrospective review I found that 30 of 36 patients (83%) had improved substantially with it, many of those to the point of having no aching most of the time. Trazodone, diphenhydramine, carisoprodol, and doxepin have similar effects on deep sleep and are also widely prescribed for sleep in FMS, but have not yet been studied in controlled blinded trials. Cyclobenzaprine and diphenhydramine are pregnancy category B and thus preferable in women who are pregnant or attempting conception. Alprazolam is pregnancy category D and so should be avoided in these patients.
Medications effective in the treatment of FMS appear to work mainly through an effect on deep sleep (Goldenberg 1986). They should be started at the lowest possible dose and increased every few days to a week to maximum relief of daytime FMS symptoms without unacceptable side effects. I allow patients to fine-tune the dose themselves. The starting doses and ranges of several medications useful in the treatment of FMS are listed in Table 1 in roughly the order I tend to try them. Amitriptyline is an effective medication for FMS but it has frequent daytime side effects attributable to its long half life such as weight gain, dry mouth, and cognitive impairment, so I usually start with the shorter-acting medications.
It is often necessary to try several different medications in succession and sometimes in combination before finding a regimen that works well. Tolerance often develops to the sedative effect of many of these, necessitating one or two dose increases after an initial good response to maintain efficacy. When switching from one medication to another, I recommend adding the second while continuing the first to try to maintain sleep quality and avoid exacerbating FMS symptoms unless problems with the first medication preclude this approach. Once improvement is noted or if side effects on the combination develop, the patient is instructed to begin tapering the first medication slowly. If it is necessary to taper the first medication while increasing the second, dose changes should not be made in both medications at the same time. Leaving a few days between an increase in the dose of one medication and a decrease in the dose of another makes it possible to tell whether it is the withdrawal of the first medication or the addition of the second that is causing any problems that develop.
Imipramine, steroids, and non-steroidal anti-inflammatory drugs (NSAIDs) have all been found to be no better than placebo for FMS (Goldenberg 1993). While NSAIDs might be expected to be helpful if only for the analgesic effect, their tendency to cause some insomnia may cancel out the expected benefit. Narcotics and benzodiazepines other than alprazolam block stage 4 sleep and so should be avoided. While they may help symptomatically, they often make the patient feel worse the next day and may prevent her from ever being able to get to the point of being pain-free most of the time. Tramadol and acetaminophen do not seem to interfere with sleep and are therefore a better choice for analgesia.
Fluoxetine was found in one study to be ineffective except to symptomatically treat associated depression (Wolfe, 1994). A second study found it effective, especially in combination with amitriptyline (Goldenberg 1996), but thi have been because fluoxetine increases amitriptyline levels which weren't monitored. A second serotonin re-uptake inhibitor, citalopram, was ineffective for FMS symptoms (Nxrregaard 1995).
There are many other unstudied "alternative" drug and herbal treatments, some of which may in the future be proven effective in controlled studies. I do not recommend these since they are as yet unproven scientifically and may have unrecognized toxicities, but I have given up trying to dissuade patients from trying them as long as it is not in place of conventional therapy.
Daily, gentle, low-impact aerobic exercise appears to be of central importance in the treatment of FMS (McCain 1988), but too much or the wrong kind of exercise may exacerbate FMS symptoms. Patients who are deconditioned should start out with just 3-5 minutes of exercise every day and gradually increase as tolerated, usually up to 20-30 minutes a day. The benefit of the exercise seems to be from its systemic effects rather than any direct effect on the exercised muscles. It works better if the patient avoids exercising the most painful muscles.
Patients should try different ways of exercising to find the best kind for them. Walking, bicycling, and the use of various types of home exercise equipment are popular. Aerobic water exercise may be best tolerated because it eliminates weight-bearing, but it is hard for patients to get to a pool every day. Water temperature is important -- patients may do poorly if the water is too cold or too warm. Water exercise is particularly useful to get patients started exercising when they can't tolerate anything else. Once their stamina improves, they should add another form of exercise on the days they don't swim.
Exercise is most effective if done in the late afternoon or early evening, perhaps because of its known effect on deep sleep. A small percentage of patients can never get up to an effective amount of exercise, but without it, few will improve much in my experience. Patients who have been exercising daily and then skip a day will usually complain of feeling worse for 2-3 days afterward, an experience which often helps convince them of the need for daily exercise.
Getting adequate sleep is essential. FMS symptoms often appear during times of sleep disruption (Saskin 1986) such as may be brought on by an injury or other pain, stress, shift work, or having to get up to attend to young children. At times just re-establishing a regular sleep scheduleenough to relieve symptoms. I have not been able to get patients who swing shifts to improve substantially unless they can get onto shifts that allow them to sleep nights and keep a consistent bedtime.
Other coexisting sleep disorders such as obstructive sleep apnea (OSA) and periodic limb movements of sleep must be identified and treated. Not infrequently a spouse's snoring will exacerbate the patient's symptoms, in which case treating the spouse's snoring or having the patient wear ear plugs will help. 44% of men with FMS have been found to also have OSA (May 1993), a potentially life-threatening disorder which is important to treat in its own right. It is important to take a sleep history in all patients with FMS, including asking the spouse about snoring, apneas, and movements at night. In resistant FMS cases, referral to a sleep disorders center for polysomnography may be helpful.
Patients must also be careful not to overdo physical activity. For example, once she is feeling better a FMS patient may try to catch up on housework she has been unable to do, but this may trigger a relapse that puts her in bed for several days. It is better to plan to spend a smaller amount of time every day at such activities until they are completed. Patients who learn to sense when they have reached their limit and stop before they get into trouble are more productive overall.
Other treatment modalities which have been shown in controlled studies to be helpful include EMG biofeedback (Ferraccioli 1989), regional sympathetic blockade (Bengtsson 1988), and cognitive behavioral therapy (Goldenberg 1991, White 1995). Many patients report that gentle massage as well as heat and rest help.
Some report that, as with migraine, certain foods appear to precipitate their symptoms. Several patients have told me that their FMS symptoms improved significantly on a low-fat weight reduction diet started to lose the weight gained from taking amitriptyline. As the benefit persisted if they continued the same diet without restricting calories, I suspect that it was something in their previous diet that was exacerbating their FMS symptoms as occurs with migraine and irritable bowel syndrome rather than the caloric restriction.
Most patients do better if they give up caffeine and other stimulants entirely. Alcohol should be avoided because of its tendency to suppress deep sleep. This is usually not a problem because most FMS patients seem to tolerate alcohol poorly to begin with. Certain symptoms such as migraine or depression can also be treated directly if treatment of the underlying disorder does not control them adequately.
FMS and myofascial pain syndrome (MPS), while probably separate entities, often coexist (Granges 1993). When they do, each needs to be treated separately. MPS is associated with trigger points which should be distinguished from the tender points of FMS. A trigger point is located over a palpable band of taut muscle and causes pain that radiates away from the point of pressure. MPS is usually treated with avoidance of activities which worsen it, myofascial release and other forms of physical therapy, and if necessary, acupuncture-like trigger point injections.
Support and education are important. Patients need to be actively involved in their treatment and to have as clear an understanding of this complicated disorder as possible. Patients often elicit less sympathy and support from family, friends, and employers than they deserve because of the lack of physical stigmata of disease. By the time they get to see someone skilled in the management of FMS, many patients will have been told by at least one other physician that there is nothing wrong with them or that it is "all in your head" which can be quite demoralizing. An understanding approach by the physician and the patient's participation in a well-run support group may have considerable therapeutic benefit.
Education, frequent follow-up visits, and reassurance help to get patients over the first few weeks of treatment. It may be difficult to convince patients to exercise when they experience fatigue and aching. It often takes two weeks or more before the beneficial effects of medication and exercise begin to outweigh their side effects. Sometimes it takes several months of trying different medications in different combinations and adjusting doses before getting it right. The physician should check on the amount and type of exercise and sleep at return visits and reinforce their importance. Patients should be warned that despite optimum treatment and good initial results, brief relapses are common, often caused by temporary sleep disturbances. The patient will do best if she "gives in to it", takes hot baths, and tries to get extra rest during a relapse. A temporary increase in medication dose may also be necessary.
A small number of patients continue to do poorly despite treatment. Severely affected patients who can't be controlled otherwise (treatment failures) need to be involved in a chronic pain program, as outpatients or if necessary inpatients. Some may need to apply for disability, which is harder to get for patients with FMS because of the lack of supporting physical or laboratory evidence, but guidelines are available (White 1995). With treatment however, the majority who were working can return to work although some may need to change jobs or get off shift work. Most patients referred to me as treatment failures never had an adequate trial of treatment.
FMS is a common, chronic, and if untreated, often disabling disorder of unknown etiology associated with neuroendocrine and immunologic changes and disordered deep sleep. Most patients can be helped with a combination of medication, exercise, and maintenance of a regular sleep schedule. Think of this condition in any patient with a complaint of aching and tiredness and look for associated symptoms and tender points to confirm the diagnosis. The common misconceptions that FMS is a psychosomatic or somatoform disorder, that it is untreatable, that it is a diagnosis of exclusion or a "wastebasket" diagnosis, and that most FMS patients are hypochondriacs or whiners are unfounded and insupportable
Drug name Starting Hrs before Usual maximum
dose (mgs) bed taken dose (mgs)
trazodone 50 0 600
cyclobenzaprine 10 1 60
alprazolam 0.5 .5-1 4
carisoprodol 350 0 1400
diphenhydramine 50 .5-1 300
amitriptyline 5 2 150
widespread pain 97.6% of patients
tenderness in > 11/18 tender points 90.1
fatigue 81.4
morning stiffness 77.0
sleep disturbance 74.6
paresthesias 62.8
headache 52.8
anxiety 47.8
dysmenorrhea history 40.6
sicca symptoms 35.8
prior depression 31.5
irritable bowel syndrome 29.6
urinary urgency 26.3
Raynaud's phenomenon 16.7
Other commonly reported symptoms include dizziness, trouble with memory and concentration, rashes, and chronic itching (unpublished observations).
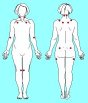
To meet ACR 1990 diagnostic criteria for fibromyalgia, digital palpation with an approximate force of 4 kgs. must produce a report of pain in at least 11 of these 18 tender points. Other areas can be tender as well. The tenderness should be focal rather than diffuse. Tender points must be present on both sides of the body, above and below the waist and in the midline. Widespread pain must have been present for at least 3 months. Some accept a diagnosis of fibromyalgia with fewer than 11 tender points if several associated symptoms from table 2 are also present (Wolfe 1989).
B Baldessarini RJ. Drugs and treatment of psychiatric disorders.
In: LS Goodman and A Gilman eds., The pharmacologic basis of
theraputics. 7th ed., New York: MacMillan, p. 413, 1985.
Bengtsson A, Bengtsson M. Regional sympathetic blocade in
primary fibromyalgia. Pain 33:161, 1988.
Bennett RM. Beyond fibromyalgia: ideas on etiology and
treatment. J Rheumatol 16(suppl 19):185, 1989.
Bennett RM et al. Low levels of somatomedin C in patients with
the fibromyalgia syndrome: a possible link between sleep and
muscle pain. Arthritis Rheum 35:1113, 1992.
Boland EW. Psychogenic rheumatism: the musculoskeletal
expression of psychoneurosis. Ann Rheum Dis 6:195, 1947.
Buchwald D et al. The chronic, active Epstein-Barr virus
infection syndrome and primary fibromyalgia. Arthritis
Rheum 30:1132, 1987.
Campbell SM et al. Clinical characteristics of fibrositis.
I. A "blinded" controlled study of symptoms and tender
points. Arthritis Rheum 26:817, 1983.
Chase TN and DL Murphy. Serotonin and central nervous system
function. Ann Rev Pharmacol 13:181, 1973.
Crofford LJ et al. Hypothalamic-pituitary-adrenal axis
perturbations in patients with fibromyalgia. Arthritis
Rheum. 37:1583-1592, 1994.
Dunne FJ and CA Dunne. Fibromyalgia syndrome and psychiatric
disorder. Br. J Hosp. Med. 54:194-197, 1995.
Ferraccioli GF et al. EMG biofeedback in fibromyalgia syndrome.
J Rheumatol 16:1013, 1989.
Gowers WR. Lumbago -- its lessons and analogues. Br Med J.
1:117, 1904.
Goldenberg DL et al. A randomized controlled trial of
amitriptyline and naproxen in the treatment of patients with
fibromyalgia. Arthritis Rheum 29:1371, 1986.
Goldenberg DL. Psychological symptoms and psychiatric diagnosis
in patients with fibromyalgia. J Rheumatol 16(suppl 19):
127, 1989.
Goldenberg DL. Fibromyalgia and chronic fatigue syndrome:
are they the same? J Musculoskel Med. 7:19, 1990.
Goldenberg DL et al. High frequency of fibromyalgia in patients
with chronic fatigue seen in a primary care practice.
Arthritis Rheum 33: 1132, 1990.
Goldenberg DL et al. The impact of cognitive-behavioral therapy
on fibromyalgia. Arthritis Rheum 34(suppl 9):S190, 1991.
Goldenberg DL. Fibromyalgia: treatment programs. J Musculoskel
Pain. 1(3/4):71, 1993.
Goldenberg D et al. A randomized, double-blind crossover trial
of fluoxetine and amitriptyline in the treatment of
fibromyalgia. Arthritis Rheum 39:1852, 1996.
Granges G, Littlejohn G. Prevalence of myofascial pain syndrome
in fibromyalgia syndrome and regional pain syndrome: a
comparative study. J Musculoskel Pain 1(2):19, 1993.
Hauri P, Hawkins DR. Alpha-delta sleep. Electroenceph Clin
Neurophysiol. 34:233, 1973.
Hobson JA. Sleep after exercise. Science 162:1503, 1968.
Jaeschke R et al. Clinical usefulness of amitriptyline in
fibromyalgia: The results of 23 N-of-1 randomized,
controlled trials. J Rheumatol 18:447-451, 1991.
May KP et al. Sleep apnea in male patients with the
fibromyalgia syndrome. Am J Med 94:505, 1993
McCain GA et al. A controlled study of the effects of a
supervised cardiovascular fitness training program on
manifestations of primary fibromyalgia. Arthritis Rheum
31:1135, 1988.
Moldofsky HD et al. Musculoskeletal symptoms and non-REM
sleep disturbance in patients with "fibrositis syndrome" and
healthy subjects. Psychosom Med. 37:341, 1975.
Moldofsky HD. A chronobiologic theory of fibromyalgia. J
Musculoskel Pain 1(3/4):49, 1993.
Moldofsky HD. Sleep, neuroimmune and neuroendocrine functions
in fibromyalgia and chronic fatigue syndrome. Adv. in
Neuroimmunol. 5:39-56, 1995.
Nxrregaard J et al. A randomized controlled trial of
citalopram in the treatment of fibromyalgia. Pain 61:
445-9, 1995.
Pellegrino MJ et al. Familial occurrence of primary
fibromyalgia. Arch Phys Med Rehab 70:61, 1989.
Russell IJ et al. Treatment of primary fibrositis/fibromyalgia
syndrome with ibuprofen and alprazolam -- a double-blind,
placebo-controlled study. Arthritis Rheum 34:552, 1991.
Russell IJ et al. Elevated cerebrospinal fluid levels of
substance P in patients with the fibromyalgia syndrome.
Arthritis Rheum 37:1593-1601, 1994.
Russell IJ. Neurochemical pathogenesis of fibromyalgia
syndrome. J Musculoskel Pain 4(1/2):61-92, 1996.
Saskin P et al. Sleep and post-traumatic rheumatic pain
modulation disorder (fibrositis syndrome). Psychosom Med
48:319, 1986.
Schroder HD et al. Muscle biopsy in fibromyalgia. J
Musculoskel Pain 1(3/4):165, 1993.
Simms RW et al. Lack of association between fibromyalgia
syndrome and abnormalities in muscle energy metabolism.
Arthritis Rheum 37:801-807, 1994.
Smythe H. Fibrositis syndrome: a historical perspective. J
Rheumatol 16(suppl 19):2, 1989.
Vestergaard-Poulsen P et al. 31P NMR spectroscopy and
electromyography during exercise and recovery in patients
with fibromyalgia. J Rheumatol 22:1544-1551, 1995.
Wallace DJ. et al. Fibromyalgia, cytokines, fatigue syndromes,
and immune regulation. In: Advances in Pain Research and
Therapy, JR Fricton and E Awad, eds., Raven Press, v.17:
227-287, 1990.
White KP et al. Work disability evaluation and the
fibromyalgia syndrome. Semin Arthritis Rheum 24:371-381,
1995.
White-KP, Nielson-WR. Cognitive behavioral treatment of
fibromyalgia syndrome: a followup assessment. J Rheumatol
22(4): 717-21, 1995.
Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin
North Am. 15:1, 1989.
Wolfe F et al. The American College of Rheumatology 1990
criteria for the classification of fibromyalgia: report of
the multicenter criteria committee. Arthritis Rheum.
33:160, 1990.
Wolfe F. Fibromyalgia: on diagnosis and certainty. J
Musculoskel Pain 1(3/4):17, 1993.
Wolfe F. The epidemiology of fibromyalgia. J Musculoskel Pain
1(3/4):137, 1993.
Wolfe F et al. A double-blind placebo controlled trial of
fluoxetine in fibromyalgia. Scand J Rheumatol 23(5):
255-9, 1994.
Yunus MB et al. A controlled study of primary fibromyalgia
syndrome: clinical features and association with other
functional syndromes. J Rheumatol 16(suppl 19):62, 1989.
Yunus MB et al. Relationship of clinical features with
psychological status in primary fibromyalgia. Arthritis
Rheum 34(1):15-21, 1991.
David A. Nye MD (nyeda@uwec.edu)
* Midelfort Clinic, Eau Claire, WI